Almurzinova Zavrish Bisembaevna , teacher of biology and chemistry MBOU “State Farm Basic Secondary School of Adamovsky District, Orenburg Region.
Subject - chemistry, grade - 9.
Educational complex: “Inorganic chemistry”, authors: G.E. Rudzitis, F.G. Feldman, Moscow, “Enlightenment”, 2014.
Level of training – basic.
Subject : “Hydrogen sulfide. Sulfides. Sulphur dioxide. Sulfurous acid and its salts." Number of hours on the topic – 1.
Lesson No. 4 in the lesson system on the topic« Oxygen and sulfur ».
Target : Based on knowledge of the structure of hydrogen sulfide and sulfur oxides, consider their properties and production, introduce students to methods for recognizing sulfides and sulfites.
Tasks:
1. Educational – study the structural features and properties of sulfur compounds (II) And(IV); become familiar with qualitative reactions to sulfide and sulfite ions.
2. Developmental – develop students’ skills in conducting experiments, observing results, analyzing and drawing conclusions.
3. Educational – developing interest in what is being studied, instilling skills in relating to nature.
Planned results : be able to describe physical and Chemical properties hydrogen sulfide, hydrosulfide acid and its salts; know methods for producing sulfur dioxide and sulfurous acid, explain the properties of sulfur compounds(II) and (IV) based on ideas about redox processes; have an idea of the effect of sulfur dioxide on the occurrence of acid rain.
Equipment : On the demonstration table: sulfur, sodium sulfide, iron sulfide, litmus solution, sulfuric acid solution, lead nitrate solution, chlorine in a cylinder closed with a stopper, a device for producing hydrogen sulfide and testing its properties, sulfur oxide (VI), oxygen gas meter, 500 ml glass, spoon for burning substances.
During the classes :
Organizing time .
We conduct a conversation on repeating the properties of sulfur:
1) what explains the presence of several allotropic modifications of sulfur?
2) what happens to the molecules: A) when vaporous sulfur is cooled. B) during long-term storage of plastic sulfur, c) when crystals precipitate from a solution of sulfur in organic solvents, for example in toluene?
3) what is the flotation method of purifying sulfur from impurities, for example from river sand, based on?
We call two students: 1) draw diagrams of molecules of various allotropic modifications of sulfur and talk about their physical properties. 2) compose reaction equations characterizing the properties of oxygen and consider them from the point of view of oxidation-reduction.
The rest of the students solve the problem: what is the mass of zinc sulfide formed during the reaction of a zinc compound with sulfur, taken with an amount of substance of 2.5 mol?
Together with the students, we formulate the lesson objective : get acquainted with the properties of sulfur compounds with oxidation states -2 and +4.
New topic : Students name compounds known to them in which sulfur exhibits these oxidation states. Chemical, electronic and structural formulas hydrogen sulfide, sulfur oxide (IV), sulfurous acid.
How can you get hydrogen sulfide? Students write down the equation for the reaction of sulfur with hydrogen and explain it from the point of view of oxidation-reduction. Then another method for producing hydrogen sulfide is considered: the exchange reaction of acids with metal sulfides. Let's compare this method with methods for producing hydrogen halides. We note that the degree of sulfur oxidation in exchange reactions does not change.
What properties does hydrogen sulfide have? In a conversation, we find out the physical properties and note the physiological effect. We determine the chemical properties by experimenting with the combustion of hydrogen sulfide in air under various conditions. What can be formed as reaction products? We consider reactions from the point of view of oxidation-reduction:
2 N 2 S+3O 2 = 2H 2 O+2SO 2
2H 2 S+O 2 =2H 2 O+2S
We draw students' attention to the fact that with complete combustion, more complete oxidation occurs (S -2 - 6 e - = S +4 ) than in the second case (S -2 - 2 e - = S 0 ).
We discuss how the process will go if chlorine is used as an oxidizing agent. We demonstrate the experience of mixing gases in two cylinders, the top of which is pre-filled with chlorine, the bottom with hydrogen sulfide. Chlorine becomes discolored and hydrogen chloride is formed. Sulfur settles on the walls of the cylinder. After this, we consider the essence of the decomposition reaction of hydrogen sulfide and lead students to the conclusion about the acidic nature of hydrogen sulfide, confirming it with experience with litmus. Then we carry out a qualitative reaction to the sulfide ion and compose the reaction equation:
Na 2 S+Pb(NO 3 ) 2 =2NaNO 3 +PbS ↓
Together with the students, we formulate the conclusion: hydrogen sulfide is only a reducing agent in redox reactions, it is acidic in nature, and its solution in water is an acid.
S 0 →S -2 ; S -2 →S 0 ; S 0 →S +4 ; S -2 →S +4 ; S 0 →H 2 S -2 → S +4 ABOUT 2.
We lead students to the conclusion that there is a genetic connection between sulfur compounds and begin a conversation about the compoundsS +4 . We demonstrate experiments: 1) obtaining sulfur oxide (IV), 2) discoloration of the fuchsin solution, 3) dissolution of sulfur oxide (IV) in water, 4) acid detection. We compose reaction equations for the experiments performed and analyze the essence of the reactions:
2SABOUT 2 + ABOUT 2 =2 SABOUT 3 ; SABOUT 2 +2H 2 S=3S+2H 2 ABOUT.
Sulfurous acid is an unstable compound, easily decomposes into sulfur oxide (IV) and water, therefore it exists only in aqueous solutions. This acid is of medium strength. It forms two rows of salts: the middle ones are sulfites (SABOUT 3 -2 ), acidic – hydrosulfites (H.S.ABOUT 3 -1 ).
We demonstrate experience: qualitative determination of sulfites, interaction of sulfites with a strong acid, which releases gasSABOUT 2 pungent odor:
TO 2 SABOUT 3 + N 2 SABOUT 4 → K 2 SABOUT 4 + N 2 O +SABOUT 2
Consolidation. Work on two options to draw up application schemes: 1 option for hydrogen sulfide, the second option for sulfur oxide (IV)
Reflection . Let's summarize the work:
What connections did we talk about today?
What properties do sulfur compounds exhibit?II) And (IV).
Name the areas of application of these compounds
VII. Homework: §11,12, exercises 3-5 (p.34)
Chemical properties
Physical properties
Under normal conditions, hydrogen sulfide is a colorless gas with a strong, characteristic odor of rotten eggs. T pl = -86 °C, T kip = -60 °C, poorly soluble in water, at 20 °C 2.58 ml of H 2 S dissolves in 100 g of water. Very toxic, if inhaled it causes paralysis, which can be fatal. In nature, it is released as part of volcanic gases and is formed during the decay of plant and animal organisms. It is highly soluble in water; when dissolved, it forms weak hydrosulfide acid.
- In an aqueous solution, hydrogen sulfide has the properties of a weak dibasic acid:
H 2 S = HS - + H + ;
HS - = S 2- + H + .
- Hydrogen sulfide burns in the air blue flame. With limited air access, free sulfur is formed:
2H 2 S + O 2 = 2H 2 O + 2S.
With excess air supply, combustion of hydrogen sulfide leads to the formation of sulfur oxide (IV):
2H 2 S + 3O 2 = 2H 2 O + 2SO 2.
- Hydrogen sulfide has reducing properties. Depending on conditions, hydrogen sulfide can be oxidized in aqueous solution to sulfur, sulfur dioxide and sulfuric acid.
For example, it decolorizes bromine water:
H 2 S + Br 2 = 2HBr + S.
interacts with chlorine water:
H 2 S + 4Cl 2 + 4H 2 O = H 2 SO 4 + 8HCl.
A stream of hydrogen sulfide can be ignited using lead dioxide, since the reaction is accompanied by a large release of heat:
3PbO 2 + 4H 2 S = 3PbS + SO 2 + 4H 2 O.
- Interaction of hydrogen sulfide with sulfur dioxide used to obtain sulfur from waste gases of metallurgical and sulfuric acid production:
SO 2 + 2H 2 S = 3S + 2H 2 O.
The formation of native sulfur during volcanic processes is associated with this process.
- When sulfur dioxide and hydrogen sulfide are simultaneously passed through an alkali solution, thiosulfate is formed:
4SO 2 + 2H 2 S + 6NaOH = 3Na 2 S 2 O 3 + 5H 2 O.
- Reaction of dilute hydrochloric acid with iron (II) sulfide
FeS + 2HCl = FeCl 2 + H 2 S
- Reaction of aluminum sulfide with cold water
Al 2 S 3 + 6H 2 O = 2Al(OH) 3 + 3H 2 S
- Direct synthesis from elements occurs when hydrogen is passed over molten sulfur:
H 2 + S = H 2 S.
- Heating a mixture of paraffin and sulfur.
1.9. Hydrogen sulfide acid and its salts
Hydrogen sulfide acid has all the properties of weak acids. It reacts with metals, metal oxides, and bases.
As a dibasic acid, it forms two types of salts - sulfides and hydrosulfides . Hydrosulfides are highly soluble in water, sulfides of alkali and alkaline earth metals as well, and sulfides of heavy metals are practically insoluble.
Sulfides of alkali and alkaline earth metals are not colored, the rest have a characteristic color, for example, sulfides of copper (II), nickel and lead - black, cadmium, indium, tin - yellow, antimony - orange.
Ionic alkali metal sulfides M 2 S have a fluorite-type structure, where each sulfur atom is surrounded by a cube of 8 metal atoms and each metal atom is surrounded by a tetrahedron of 4 sulfur atoms. MS-type sulfides are characteristic of alkaline earth metals and have a sodium chloride-type structure, where each metal and sulfur atom is surrounded by an octahedron of atoms of a different type. As the covalent nature of the metal–sulfur bond increases, structures with lower coordination numbers are realized.
Sulfides of non-ferrous metals are found in nature as minerals and ores and serve as raw materials for the production of metals.
Hydrogen sulfide is present in artificial gases . It can also be part of some natural gases. Hydrogen sulfide (H 2 S) is a colorless gas with strong specific smell. Hydrogen sulfide heavier air. Its density is r 0 = 1.539 kg/m 3. Hydrogen sulfide is strong nerve gas , and is also irritating to the respiratory tract and eyes. The maximum permissible concentration of H 2 S is 0.01 mg/m 3. When hydrogen sulfide burns, sulfur dioxide SO 2 is formed, i.e. the following reaction occurs:
2H 2 S + 3O 2 = 2SO 2 + 2H 2 O
The higher calorific value of sulfur dioxide is Qv = 25.727 MJ/m 3, lowest – 23.715 MJ/m 3 .
Sulfur dioxide has a very large flammable area. So, the lower limit is 4.3% vol., the upper 45.5% vol. Its ignition temperature in air is 290...487 0 WITH.
Working in areas with high levels of sulfur dioxide may result in bronchitis, shortness of breath and partial loss of consciousness . The maximum permissible concentration of sulfur dioxide is 0.02 mg/m 3 .
Victims of hydrogen sulfide poisoning should be given first aid. It is necessary to ensure access to fresh air and, if necessary, carry out artificial respiration . In case of eye damage, it is necessary to transport the victim to a dark room and drip the eyes with a mixture of novocaine and adrenaline.
Victims of sulfur dioxide poisoning should wash their nose and eyes with a soda solution. If you have a suffocating cough, use codeine and alkaline inhalation .
Carbon disulfide
Carbon disulfide is usually present in pyrolysis gases, which belong to the group of artificial gases. It is a colorless liquid with a specific odor. Its density at 20 0 WITH leaves 2.263 kg/m 3. Carbon disulfide vapor is more than 2.5 times heavier than air. When carbon disulfide burns, sulfur dioxide and carbon dioxide are formed:
CS 2 + 3O 2 = CO 2 + 2SO 2 (5.6)
The flammability limits of carbon disulfide in air are: lower - 1.25% vol., upper - 50% vol.
Inhalation of high concentrations of carbon disulfide vapor has a narcotic effect on the human body. Long-term inhalation of small concentrations of carbon disulfide leads to illness nervous system. The maximum permissible concentration of carbon disulfide in the working area of industrial premises is 0.01 mg/l.
First aid for carbon disulfide poisoning is to rinse the nose and eyes with a soda solution.
Ammonia
It is usually contained in pyrolysis gases obtained during the high-temperature distillation of coal. On the one hand, ammonia is a valuable product, but on the other hand, it is quite toxic. According to its properties, ammonia is a colorless gas with a very pungent odor. Only 10% of the ammonia solution in water is ammonia. Short-term inhalation of high concentrations leads to severe watering and pain in the eyes, and also causes attacks of suffocation, coughing, dizziness and vomiting. In addition, at significant concentrations, circulatory disturbances and death from heart failure may also occur. Extremely extra conc. ammonia in the air of industrial premises is 0.02 mg/l. Complications of the consequences of poisoning, even at low concentrations of ammonia, can occur when combined with hydrogen sulfide. This can lead to loss of smell and cause chronic catarrh of the respiratory tract. In case of acute ammonia poisoning, it is necessary for the victim to inhale acetic acid vapor and a 10% solution of methanol in chloroform.
Hydrogen cyanide
Hydrogen cyanide is part of artificial gases, mainly pyrolysis gases. It is formed as a result of the interaction of ammonia with hot coke. The amount of hydrogen cyanide formed depends on a number of factors: temperature, humidity of the coal, and its nitrogen content. According to its physicochemical properties, hydrogen cyanide is a liquid that has a specific odor (the smell of bitter almonds).
Sulfur– element of the 3rd period and VIA group of the Periodic System, serial number 16, refers to chalcogens. The electronic formula of the atom is [ 10 Ne]3s 2 3p 4, the characteristic oxidation states are 0, -II, +IV and +VI, the S VI state is considered stable.
Scale of sulfur oxidation states:
The electronegativity of sulfur is 2.60 and is characterized by non-metallic properties. In hydrogen and oxygen compounds it is found in various anions and forms oxygen-containing acids and their salts, binary compounds.
In nature - fifteenth element by chemical abundance (seventh among non-metals). It is found in free (native) and bound form. A vital element for higher organisms.
Sulfur S. Simple substance. Yellow crystalline (α‑rhombic and β‑monoclinic,
at 95.5 °C) or amorphous (plastic). At the nodes of the crystal lattice there are S 8 molecules (non-planar rings of the “crown” type), amorphous sulfur consists of S n chains. A low-melting substance, the viscosity of the liquid passes through a maximum at 200 °C (breakdown of S 8 molecules, interweaving of S n chains). The pair contains molecules S 8, S 6, S 4, S 2. At 1500 °C, monoatomic sulfur appears (in chemical equations, for simplicity, any sulfur is depicted as S).
Sulfur is insoluble in water and under normal conditions does not react with it; it is highly soluble in carbon disulfide CS 2.
Sulfur, especially powdered sulfur, is highly active when heated. Reacts as an oxidizing agent with metals and non-metals:

but as reducing agent– with fluorine, oxygen and acids (boiling):

Sulfur undergoes dismutation in alkali solutions:
3S 0 + 6KOH (conc.) = 2K 2 S ‑II + K 2 S IV O 3 + 3H 2 O
At high temperature(400 °C) sulfur displaces iodine from hydrogen iodide:
S + 2HI (g) = I 2 + H 2 S,
but in solution the reaction goes in the opposite direction:
I 2 + H 2 S (p) = 2 HI + S↓
Receipt: V industry smelted from natural deposits of native sulfur (using water vapor), released during desulfurization of coal gasification products.
Sulfur is used for the synthesis of carbon disulfide, sulfuric acid, sulfur (vat) dyes, during the vulcanization of rubber, as a means of protecting plants from powdery mildew, for the treatment of skin diseases.
Hydrogen sulfide H 2 S. Anoxic acid. A colorless gas with a suffocating odor, heavier than air. The molecule has the structure of a doubly incomplete tetrahedron [::S(H) 2 ]
(sp 3 -hybridization, valet angle H – S–H is far from tetrahedral). Unstable when heated above 400 °C. Slightly soluble in water (2.6 l/1 l H 2 O at 20 °C), saturated decimolar solution (0.1 M, “hydrogen sulfide water”). A very weak acid in solution, practically does not dissociate in the second stage to S 2‑ ions (the maximum concentration of S 2‑ is 1 10 ‑ 13 mol/l). When exposed to air, the solution becomes cloudy (the inhibitor is sucrose). Neutralized by alkalis, but not completely by ammonia hydrate. Strong reducing agent. Enters into ion exchange reactions. A sulfiding agent precipitates differently colored sulfides with very low solubility from solution.
Qualitative reactions– precipitation of sulfides, as well as incomplete combustion of H 2 S with the formation of a yellow sulfur deposit on a cold object brought into the flame (porcelain spatula). A by-product of oil, natural and coke oven gas refining.
It is used in the production of sulfur, inorganic and organic sulfur-containing compounds as an analytical reagent. Extremely poisonous. Equations of the most important reactions:


Receipt: V industry– direct synthesis:
H 2 + S = H2S(150–200 °C)
or by heating sulfur with paraffin;
V laboratories– displacement from sulfides with strong acids
FeS + 2НCl (conc.) = FeCl 2 + H2S
or complete hydrolysis of binary compounds:
Al 2 S 3 + 6H 2 O = 2Al(OH) 3 ↓ + 3 H2S
Sodium sulfide Na 2 S. Oxygen-free salt. White, very hygroscopic. Melts without decomposition, thermally stable. It is highly soluble in water, hydrolyzes at the anion, and creates a highly alkaline environment in solution. When exposed to air, the solution becomes cloudy (colloidal sulfur) and turns yellow (polysulfide color). Typical reducer. Adds sulfur. Enters into ion exchange reactions.
Qualitative reactions on the S 2‑ ion – precipitation of differently colored metal sulfides, of which MnS, FeS, ZnS decompose into HCl (diluted).
It is used in the production of sulfur dyes and cellulose, for removing hair from hides when tanning leather, as a reagent in analytical chemistry.
Equations of the most important reactions:
Na 2 S + 2НCl (diluted) = 2NaCl + H 2 S
Na 2 S + 3H 2 SO 4 (conc.) = SO 2 + S↓ + 2H 2 O + 2NaHSO 4 (up to 50 °C)
Na 2 S + 4HNO 3 (conc.) = 2NO + S↓ + 2H 2 O + 2NaNO 3 (60 °C)
Na 2 S + H 2 S (saturated) = 2NaHS
Na 2 S (t) + 2O 2 = Na 2 SO 4 (above 400 °C)
Na 2 S + 4H 2 O 2 (conc.) = Na 2 SO 4 + 4H 2 O
S 2‑ + M 2+ = MnS (tel.)↓; FeS (black)↓; ZnS (white)↓
S 2‑ + 2Ag + = Ag 2 S (black)↓
S 2‑ + M 2+ = СdS (yellow)↓; PbS, CuS, HgS (black)↓
3S 2‑ + 2Bi 3+ = Bi 2 S 3 (cor. – black)↓
3S 2‑ + 6H 2 O + 2M 3+ = 3H 2 S + 2M(OH) 3 ↓ (M = Al, Cr)
Receipt V industry– calcination of the mineral mirabilite Na 2 SO 4 10H 2 O in the presence of reducing agents:
Na 2 SO 4 + 4H 2 = Na 2 S + 4H 2 O (500 °C, cat. Fe 2 O 3)
Na 2 SO 4 + 4С (coke) = Na 2 S + 4СО (800–1000 °C)
Na 2 SO 4 + 4СО = Na 2 S + 4СО 2 (600–700 °C)
Aluminum sulfide Al 2 S 3. Oxygen-free salt. White, the Al–S bond is predominantly covalent. Melts without decomposition under excess pressure N 2, easily sublimes. Oxidizes in air when heated. It is completely hydrolyzed by water and does not precipitate from solution. Decomposes with strong acids. Used as a solid source of pure hydrogen sulfide. Equations of the most important reactions:
Al 2 S 3 + 6H 2 O = 2Al(OH) 3 ↓ + 3H 2 S (pure)
Al 2 S 3 + 6HCl (diluted) = 2AlCl 3 + 3H 2 S
Al 2 S 3 + 24HNO 3 (conc.) = Al 2 (SO 4) 3 + 24NO 2 + 12H 2 O (100 °C)
2Al 2 S 3 + 9O 2 (air) = 2Al 2 O 3 + 6SO 2 (700–800 °C)
Receipt: interaction of aluminum with molten sulfur in the absence of oxygen and moisture:
2Al + 3S = AL 2 S 3(150–200 °C)
Iron (II) sulfide FeS. Oxygen-free salt. Black-gray with green tint, refractory, decomposes when heated in a vacuum. In wet sensitive to oxygen in the air. Insoluble in water. Does not precipitate when solutions of iron(II) salts are saturated with hydrogen sulfide. Decomposes with acids. It is used as a raw material in the production of cast iron, a solid source of hydrogen sulfide.
The iron(III) compound Fe 2 S 3 is not known (not obtained).
Equations of the most important reactions:

Receipt:
Fe + S = FeS(600 °C)
Fe 2 O 3 + H 2 + 2H 2 S = 9 FeS+ 3H 2 O (700‑1000 °C)
FeCl 2 + 2NH 4 HS (g) = FeS↓ + 2NH 4 Cl + H 2 S
Iron disulfide FeS 2. Binary connection. It has the ionic structure Fe 2+ (–S – S–) 2‑ . Dark yellow, thermally stable, decomposes when heated. Insoluble in water, does not react with dilute acids and alkalis. Decomposes by oxidizing acids and is fired in air. It is used as a raw material in the production of cast iron, sulfur and sulfuric acid, and a catalyst in organic synthesis. Ore minerals found in nature pyrite And Marcasite.
Equations of the most important reactions:
FeS 2 = FeS + S (above 1170 °C, vacuum)
2FeS 2 + 14H 2 SO 4 (conc., horizontal) = Fe 2 (SO 4) 3 + 15SO 2 + 14H 2 O
FeS 2 + 18HNO 3 (conc.) = Fe(NO 3) 3 + 2H 2 SO 4 + 15NO 2 + 7H 2 O
4FeS 2 + 11O 2 (air) = 8SO 2 + 2Fe 2 O 3 (800 °C, roasting)
Ammonium hydrosulfide NH 4 HS. An oxygen-free acidic salt. White, melts under excess pressure. Very volatile, thermally unstable. It oxidizes in air. It is highly soluble in water, hydrolyzes into the cation and anion (predominates), creates an alkaline environment. The solution turns yellow in air. Decomposes with acids and adds sulfur in a saturated solution. It is not neutralized by alkalis, the average salt (NH 4) 2 S does not exist in solution (for the conditions for obtaining the average salt, see the section “H 2 S”). It is used as a component of photographic developers, as an analytical reagent (sulfide precipitator).
Equations of the most important reactions:
NH 4 HS = NH 3 + H 2 S (above 20 °C)
NH 4 HS + HCl (diluted) = NH 4 Cl + H 2 S
NH 4 HS + 3HNO 3 (conc.) = S↓ + 2NO 2 + NH 4 NO 3 + 2H 2 O
2NH 4 HS (saturated H 2 S) + 2CuSO 4 = (NH 4) 2 SO 4 + H 2 SO 4 + 2CuS↓
Receipt: saturation of a concentrated solution of NH 3 with hydrogen sulfide:
NH 3 H 2 O (conc.) + H 2 S (g) = NH 4 HS+ H 2 O
In analytical chemistry, a solution containing equal amounts of NH 4 HS and NH 3 H 2 O is conventionally considered a solution of (NH 4) 2 S and the formula of the average salt is used in writing the reaction equations, although ammonium sulfide is completely hydrolyzed in water to NH 4 HS and NH 3H2O.
Sulfur dioxide. Sulfites
Sulfur dioxide SO2. Acidic oxide. Colorless gas with a pungent odor. The molecule has the structure of an incomplete triangle [: S(O) 2 ] (sp 2 - hybridization), contains σ, π bonds S=O. Easily liquefied, thermally stable. Highly soluble in water (~40 l/1 l H 2 O at 20 °C). Forms a polyhydrate with the properties of a weak acid; dissociation products are HSO 3 - and SO 3 2 - ions. The HSO 3 ion has two tautomeric forms - symmetrical(non-acidic) with a tetrahedral structure (sp 3 -hybridization), which predominates in the mixture, and asymmetrical(acidic) with the structure of an incomplete tetrahedron [: S(O) 2 (OH)] (sp 3 -hybridization). The SO 3 2‑ ion is also tetrahedral [: S(O) 3 ].
Reacts with alkalis, ammonia hydrate. A typical reducing agent, weak oxidizing agent.
Qualitative reaction– discoloration of yellow-brown “iodine water”. Intermediate product in the production of sulfites and sulfuric acid.
It is used for bleaching wool, silk and straw, canning and storing fruits, as a disinfectant, antioxidant, and refrigerant. Poisonous.
The compound of composition H 2 SO 3 (sulfurous acid) is unknown (does not exist).
Equations of the most important reactions:

Solubility in water and acidic properties:

Receipt: in industry - combustion of sulfur in air enriched with oxygen, and, to a lesser extent, roasting of sulfide ores (SO 2 - associated gas when roasting pyrite):
S + O 2 = SO 2(280–360 °C)
4FeS 2 + 11O 2 = 2Fe 2 O 3 + 8 SO 2(800 °C, firing)
in the laboratory - displacement of sulfites with sulfuric acid:
BaSO 3 (t) + H 2 SO 4 (conc.) = BaSO 4 ↓ + SO 2 + H 2 O
Sodium sulfite Na 2 SO 3. Oxosol. White. When heated in air, it decomposes without melting and melts under excess pressure of argon. When wet and in solution, it is sensitive to atmospheric oxygen. It is highly soluble in water and hydrolyzes at the anion. Decomposes with acids. Typical reducer.
Qualitative reaction on the SO 3 2‑ ion - the formation of a white precipitate of barium sulfite, which is transferred into solution with strong acids (HCl, HNO 3).
It is used as a reagent in analytical chemistry, a component of photographic solutions, and a chlorine neutralizer for bleaching fabrics.
Equations of the most important reactions:

Receipt:
Na 2 CO 3 (conc.) + SO 2 = Na2SO3+CO2
Sulfuric acid. Sulfates
Sulfuric acid H 2 SO 4. Oxoacid. Colorless liquid, very viscous (oily), very hygroscopic. The molecule has a distorted tetrahedral structure (sp 3 -hybridization), contains covalent σ-bonds S – OH and σπ-bonds S=O. The SO 4 2‑ ion has a regular tetrahedral structure. It has a wide temperature range of the liquid state (~300 degrees). Partially decomposes when heated above 296 °C. It is distilled in the form of an azeotropic mixture with water (mass fraction of acid 98.3%, boiling point 296–340 °C), and with stronger heating it decomposes completely. Unlimitedly miscible with water (with strong exo‑effect). Strong acid in solution, neutralized by alkalis and ammonia hydrate. Converts metals into sulfates (with an excess of concentrated acid under normal conditions, soluble hydrosulfates are formed), but the metals Be, Bi, Co, Fe, Mg and Nb are passivated in concentrated acid and do not react with it. Reacts with basic oxides and hydroxides, decomposes salts of weak acids. A weak oxidizing agent in a dilute solution (due to H I), a strong oxidizing agent in a concentrated solution (due to S VI). It dissolves SO 3 well and reacts with it (a heavy oily liquid is formed - oleum, contains H 2 S 2 O 7).
Qualitative reaction on the SO 4 2‑ ion – precipitation of white barium sulfate BaSO 4 (the precipitate is not transferred into solution by hydrochloric and nitric acids, unlike the white precipitate BaSO 3).
Used in the production of sulfates and other sulfur compounds, mineral fertilizers, explosives, dyes and medicines, in organic synthesis, for the “opening” (the first stage of processing) of industrially important ores and minerals, during the purification of petroleum products, the electrolysis of water, as an electrolyte for lead batteries. Toxic, causes skin burns. Equations of the most important reactions:

Receipt V industry:
a) synthesis of SO 2 from sulfur, sulfide ores, hydrogen sulfide and sulfate ores:
S + O 2 (air) = SO 2(280–360 °C)
4FeS 2 + 11O 2 (air) = 8 SO 2+ 2Fe 2 O 3 (800 °C, firing)
2H 2 S + 3O 2 (g) = 2 SO 2+ 2H 2 O (250–300 °C)
CaSO 4 + C (coke) = CaO + SO 2+ CO (1300–1500 °C)
b) conversion of SO 2 to SO 3 in a contact apparatus:
c) synthesis of concentrated and anhydrous sulfuric acid:
H 2 O (dil. H 2 SO 4) + SO 3 = H2SO4(conc., anhydrous)
(the absorption of SO 3 with pure water to produce H 2 SO 4 is not carried out due to the strong heating of the mixture and the reverse decomposition of H 2 SO 4, see above);
d) synthesis oleum– a mixture of anhydrous H 2 SO 4, disulfuric acid H 2 S 2 O 7 and excess SO 3. Dissolved SO 3 guarantees the anhydrity of oleum (when water enters, H 2 SO 4 is immediately formed), which allows it to be safely transported in steel tanks.
Sodium sulfate Na 2 SO 4. Oxosol. White, hygroscopic. Melts and boils without decomposition. Forms crystalline hydrate (mineral mirabilite), easily losing water; technical name Glauber's salt. It is highly soluble in water and does not hydrolyze. Reacts with H 2 SO 4 (conc.), SO 3 . It is reduced by hydrogen and coke when heated. Enters into ion exchange reactions.
Used in the production of glass, cellulose and mineral paints, as medicine. Contained in the brine of salt lakes, in particular in the Kara-Bogaz-Gol Bay of the Caspian Sea.
Equations of the most important reactions:

Potassium hydrogen sulfate KHSO 4. Acid oxo salt. White, hygroscopic, but does not form crystalline hydrates. When heated, it melts and decomposes. It is highly soluble in water; the anion undergoes dissociation in solution; the solution environment is strongly acidic. Neutralized by alkalis.
It is used as a component of fluxes in metallurgy, component mineral fertilizers.
Equations of the most important reactions:
2KHSO 4 = K 2 SO 4 + H 2 SO 4 (up to 240 °C)
2KHSO 4 = K 2 S 2 O 7 + H 2 O (320–340 °C)
KHSO 4 (dil.) + KOH (conc.) = K 2 SO 4 + H 2 O KHSO 4 + KCl = K 2 SO 4 + HCl (450–700 °C)
6KHSO 4 + M 2 O 3 = 2KM(SO 4) 2 + 2K 2 SO 4 + 3H 2 O (350–500 °C, M = Al, Cr)
Receipt: treatment of potassium sulfate with concentrated (more than 6O%) sulfuric acid in the cold:
K 2 SO 4 + H 2 SO 4 (conc.) = 2 KHSO 4
Calcium sulfate CaSO 4. Oxosol. White, very hygroscopic, refractory, decomposes when heated. Natural CaSO 4 occurs as a very common mineral gypsum CaSO 4 2H 2 O. At 130 °C, gypsum loses some of the water and turns into burnt (plaster) gypsum 2CaSO 4 H 2 O (technical name alabaster). Completely dehydrated (200 °C) gypsum corresponds to the mineral anhydrite CaSO4. Slightly soluble in water (0.206 g/100 g H 2 O at 20 °C), solubility decreases when heated. Reacts with H 2 SO 4 (conc.). Restored by coke during fusion. Determines most of the “permanent” hardness of fresh water (see 9.2 for details).
Equations of the most important reactions: 100–128 °C

It is used as a raw material in the production of SO 2, H 2 SO 4 and (NH 4) 2 SO 4, as a flux in metallurgy, and as a paper filler. A binder made from burnt gypsum building mixture“sets” faster than a mixture based on Ca(OH) 2. Hardening is ensured by the binding of water, the formation of gypsum in the form of a stone mass. Burnt gypsum is used to make plaster casts, architectural and decorative forms and products, partition slabs and panels, and stone floors.
Aluminum-potassium sulfate KAl(SO 4) 2. Double oxo salt. White, hygroscopic. Decomposes when heated strongly. Forms crystalline hydrate - potassium alum. Moderately soluble in water, hydrolyzed by aluminum cation. Reacts with alkalis, ammonia hydrate.
It is used as a mordant for dyeing fabrics, a leather tanning agent, a coagulant for purifying fresh water, a component of compositions for sizing paper, and an external hemostatic agent in medicine and cosmetology. It is formed by the joint crystallization of aluminum and potassium sulfates.
Equations of the most important reactions:


Chromium(III) sulfate - potassium KCr(SO 4) 2. Double oxo salt. Red (hydrate dark purple, technical name chromium-potassium alum). When heated, it decomposes without melting. It is highly soluble in water (the gray-blue color of the solution corresponds to aqua complex 3+), hydrolyzes at the chromium(III) cation. Reacts with alkalis, ammonia hydrate. Weak oxidizing and reducing agent. Enters into ion exchange reactions.
Qualitative reactions on the Cr 3+ ion – reduction to Cr 2+ or oxidation to yellow CrO 4 2‑.
It is used as a leather tanning agent, a mordant for dyeing fabrics, and a reagent in photography. It is formed by the joint crystallization of chromium(III) and potassium sulfates. Equations of the most important reactions:


Manganese (II) sulfate MnSO 4 . Oxosol. White, melts and decomposes when heated. Crystalline hydrate MnSO 4 5H 2 O – red-pink, technical name manganese sulfate. It is highly soluble in water; the light pink (almost colorless) color of the solution corresponds to aquacomplex 2+; hydrolyzes at the cation. Reacts with alkalis, ammonia hydrate. Weak reducing agent, reacts with typical (strong) oxidizing agents.
Qualitative reactions on the Mn 2+ ion – commutation with the MnO 4 ion and the disappearance of the violet color of the latter, oxidation of Mn 2+ to MnO 4 and the appearance of a violet color.
It is used for the production of Mn, MnO 2 and other manganese compounds, as a microfertilizer and analytical reagent.
Equations of the most important reactions:

Receipt:
2MnO 2 + 2H 2 SO 4 (conc.) = 2 MnSO4+ O 2 + 2H 2 O (100 °C)
Iron (II) sulfate FeSO 4 . Oxosol. White (light green hydrate, technical name inkstone), hygroscopic. Decomposes when heated. It is highly soluble in water and is slightly hydrolyzed by the cation. It is quickly oxidized in solution by atmospheric oxygen (the solution turns yellow and becomes cloudy). Reacts with oxidizing acids, alkalis, and ammonia hydrate. Typical reducer.
It is used as a component of mineral paints, electrolytes in electroplating, a wood preservative, a fungicide, and a medicine against anemia. In the laboratory it is often taken in the form of a double salt Fe(NH 4) 2 (SO 4) 2 6H 2 O ( Mohr's salt), more resistant to air.
Equations of the most important reactions:

Receipt:
Fe + H 2 SO 4 (diluted) = FeSO4+H2
FeCO 3 + H 2 SO 4 (diluted) = FeSO4+ CO 2 + H 2 O
7.4. Non-metals VA‑group
Nitrogen. Ammonia
Nitrogen– element of the 2nd period and VA group of the Periodic system, serial number 7. Electronic formula of the atom [ 2 He]2s 2 2p 3, characteristic oxidation states 0, ‑III, +III and +V, less often +II, +IV and etc.; the N v state is considered relatively stable.
Scale of nitrogen oxidation states:

Nitrogen has a high electronegativity (3.07), third after F and O. It exhibits typical non-metallic (acidic) properties. Forms various oxygen-containing acids, salts and binary compounds, as well as the ammonium cation NH 4 + and its salts.
In nature - seventeenth by chemical abundance element (ninth among non-metals). A vital element for all organisms.
Nitrogen N 2. Simple substance. It consists of non-polar molecules with a very stable σππ-bond N ≡ N, this explains the chemical inertness of nitrogen under normal conditions. A colorless, tasteless and odorless gas that condenses into a colorless liquid (unlike O2).
Main component of air: 78.09% by volume, 75.52% by mass. Nitrogen boils away from liquid air before oxygen O2. Slightly soluble in water (15.4 ml/1 l H 2 O at 20 ° C), the solubility of nitrogen is less than that of oxygen.
At room temperature, N2 reacts only with lithium (in a humid atmosphere), forming lithium nitride Li3N; nitrides of other elements are synthesized with strong heating:
N 2 + 3Mg = Mg 3 N 2 (800 °C)
In an electrical discharge, N2 reacts with fluorine and, to a very small extent, with oxygen:
The reversible reaction to produce ammonia occurs at 500 °C, under pressure up to 350 atm and always in the presence of a catalyst (Fe/F 2 O 3 /FeO, in the laboratory Pt):
According to Le Chatelier's principle, an increase in ammonia yield should occur with increasing pressure and decreasing temperature. However, the reaction rate at low temperatures is very low, so the process is carried out at 450–500 °C, achieving a 15% ammonia yield. Unreacted N 2 and H 2 are returned to the reactor and thereby increase the degree of reaction.
Nitrogen is chemically passive in relation to acids and alkalis and does not support combustion.
Receipt V industry– fractional distillation of liquid air or removal of oxygen from air by chemical means, for example, by the reaction 2C (coke) + O 2 = 2CO when heated. In these cases, nitrogen is obtained, which also contains impurities of noble gases (mainly argon).
IN laboratories small amounts of chemically pure nitrogen can be obtained by the commutation reaction with moderate heating:
N ‑III H 4 N III O 2(t) = N 2 0 + 2H 2 O (60–70 °C)
NH 4 Cl (p) + KNO 2 (p) = N 2 0 + KCl + 2H 2 O (100 °C)
It is used for the synthesis of ammonia, nitric acid and other nitrogen-containing products, as an inert medium for chemical and metallurgical processes and storage of flammable substances.
Ammonia NH3. Binary compound, the oxidation state of nitrogen is – III. Colorless gas with a sharp characteristic odor. The molecule has the structure of an incomplete tetrahedron [: N(H) 3)] (sp 3 -hybridization). The presence of a donor pair of electrons on the sp 3 -hybrid orbital of nitrogen in the NH 3 molecule determines the characteristic reaction of addition of a hydrogen cation, which results in the formation of a cation ammonium NH4+. It liquefies under excess pressure at room temperature. In the liquid state, it is associated through hydrogen bonds. Thermally unstable. Highly soluble in water (more than 700 l/1 l H 2 O at 20 °C); the proportion in the saturated solution is = 34% by mass and = 99% by volume, pH = 11.8.
Very reactive, prone to addition reactions. Cr reacts in oxygen, reacts with acids. It exhibits reducing (due to N‑III) and oxidizing (due to H I) properties. It is dried only with calcium oxide.
Qualitative reactions– formation of white “smoke” upon contact with gaseous HCl, blackening of a piece of paper moistened with a solution of Hg 2 (NO 3) 2.
An intermediate product in the synthesis of HNO 3 and ammonium salts. Used in the production of soda, nitrogen fertilizers, dyes, explosives; liquid ammonia is a refrigerant. Poisonous.
Equations of the most important reactions:

Receipt: V laboratories– displacement of ammonia from ammonium salts when heated with soda lime (NaOH + CaO):
or boiling an aqueous solution of ammonia and then drying the gas.
IN industry ammonia is synthesized from nitrogen (see) with hydrogen. Produced by industry either in liquefied form or in the form of a concentrated aqueous solution under the technical name ammonia water.
Ammonia hydrate NH 3 H 2 O. Intermolecular connection. White, in the crystal lattice - molecules NH 3 and H 2 O, connected by a weak hydrogen bond H 3 N ... HON. Present in an aqueous solution of ammonia, a weak base (dissociation products - NH 4 ‑ cation and OH ‑ anion). The ammonium cation has a regular tetrahedral structure (sp 3 hybridization). Thermally unstable, completely decomposes when the solution is boiled. Neutralized by strong acids. Shows reducing properties (due to N III) in a concentrated solution. Enters into ion exchange and complexation reactions.
Qualitative reaction– formation of white “smoke” upon contact with gaseous HCl.
It is used to create a slightly alkaline environment in solution during the precipitation of amphoteric hydroxides.
A 1M ammonia solution contains mainly NH 3 H 2 O hydrate and only 0.4% NH 4 + and OH - ions (due to hydrate dissociation); Thus, the ionic “ammonium hydroxide NH 4 OH” is practically not contained in the solution, and there is no such compound in the solid hydrate. Equations of the most important reactions:
NH 3 H 2 O (conc.) = NH 3 + H 2 O (boiling with NaOH)
NH 3 H 2 O + HCl (diluted) = NH 4 Cl + H 2 O
3(NH 3 H 2 O) (conc.) + CrCl 3 = Cr(OH) 3 ↓ + 3NH 4 Cl
8(NH 3 H 2 O) (conc.) + ZBr 2 (p) = N 2 + 6NH 4 Br + 8H 2 O (40–50 °C)
2(NH 3 H 2 O) (conc.) + 2KMnO 4 = N 2 + 2MnO 2 ↓ + 4H 2 O + 2KOH
4(NH 3 H 2 O) (conc.) + Ag2O= 2OH + 3H2O
4(NH 3 H 2 O) (conc.) + Cu(OH) 2 + (OH) 2 + 4H 2 O
6(NH 3 H 2 O) (conc.) + NiCl 2 = Cl 2 + 6H 2 O
A dilute ammonia solution (3–10%) is often called ammonia(the name was invented by alchemists), and the concentrated solution (18.5–25%) - ammonia water(produced by industry).
Related information.
Chemistry tutor
Continuation. See in No. 22/2005;
3, 4, 7, 10, 11, 21/2007;
2, 7, 11, 18, 19, 21/2008;
1, 3, 10/2009
1, 2, 3, 5, 6, 8, 9, 11, 13, 15, 16, 18, 22/2006;
LESSON 30
10th grade (first year of study)
Sulfur and its compounds
1. Position in the table of D.I. Mendeleev, structure of the atom.
2. Origin of the name.
3. Physical properties.
4. Chemical properties.
5. Being in nature.
6. Basic methods of obtaining.
7. The most important sulfur compounds (hydrogen sulfide, hydrosulfide acid and its salts; sulfur dioxide, sulfurous acid and its salts; sulfur trioxide, sulfuric acid and its salts). In the periodic table, sulfur is in the main subgroup of group VI (chalcogen subgroup). Electronic formula of sulfur 1 2 2In the periodic table, sulfur is in the main subgroup of group VI (chalcogen subgroup). Electronic formula of sulfur 1 2 s 6 3In the periodic table, sulfur is in the main subgroup of group VI (chalcogen subgroup). Electronic formula of sulfur 1 2 s p 4, this R
-element. Depending on its state, sulfur can exhibit valency II, IV or VI: In the periodic table, sulfur is in the main subgroup of group VI (chalcogen subgroup). Electronic formula of sulfur 1 2 2In the periodic table, sulfur is in the main subgroup of group VI (chalcogen subgroup). Electronic formula of sulfur 1 2 2s 6 3In the periodic table, sulfur is in the main subgroup of group VI (chalcogen subgroup). Electronic formula of sulfur 1 2 3s 4 3S: 1 d
0 (valence II), In the periodic table, sulfur is in the main subgroup of group VI (chalcogen subgroup). Electronic formula of sulfur 1 2 2In the periodic table, sulfur is in the main subgroup of group VI (chalcogen subgroup). Electronic formula of sulfur 1 2 2s 6 3In the periodic table, sulfur is in the main subgroup of group VI (chalcogen subgroup). Electronic formula of sulfur 1 2 3s 3 3S: 1 S*: 1
1 (valence IV), In the periodic table, sulfur is in the main subgroup of group VI (chalcogen subgroup). Electronic formula of sulfur 1 2 2In the periodic table, sulfur is in the main subgroup of group VI (chalcogen subgroup). Electronic formula of sulfur 1 2 2s 6 3In the periodic table, sulfur is in the main subgroup of group VI (chalcogen subgroup). Electronic formula of sulfur 1 1 3s 3 3S: 1 S**: 1
2 (valency VI).
Sulfur The characteristic oxidation states of sulfur are –2, +2, +4, +6 (in disulfides containing a bridged –S–S– bond (for example, FeS 2), the oxidation state of sulfur is –1); in compounds it is part of anions, with more electronegative elements – part of cations, for example:
– an element with high electronegativity, exhibits non-metallic (acidic) properties. It has four stable isotopes with mass numbers 32, 33, 34 and 36. Natural sulfur is 95% composed of the 32 S isotope. Russian name sulfur comes from the Sanskrit word cira – light yellow, the color of natural sulfur. Latin name sulfur
translated as "flammable powder". 1
PHYSICAL STRUCTURES allotropic modifications: rhombic(-sulfur), monoclinic(-sulfur) and plastic, or rubbery. Orthorhombic sulfur is most stable under normal conditions, and monoclinic sulfur is stable above 95.5 °C. Both of these allotropic modifications have a molecular crystal lattice built from molecules of the composition S 8 located in space in the form of a crown; atoms are connected by single covalent bonds. The difference between rhombic and monoclinic sulfur is that in the crystal lattice the molecules are packed differently.
If rhombic or monoclinic sulfur is heated to its boiling point (444.6 °C) and the resulting liquid is poured into cold water, then plastic sulfur is formed, its properties reminiscent of rubber. Plastic sulfur consists of long zigzag chains. This allotropic modification is unstable and spontaneously transforms into one of the crystalline forms.
Rhombic sulfur is a yellow crystalline solid; does not dissolve in water (and is not wetted), but is highly soluble in many organic solvents (carbon disulfide, benzene, etc.). Sulfur has very poor electrical and thermal conductivity. The melting point of orthorhombic sulfur is +112.8 °C; at a temperature of 95.5 °C, orthorhombic sulfur becomes monoclinic:
![]()
Chemical properties
In terms of its chemical properties, sulfur is a typical active non-metal. In reactions it can be both an oxidizing agent and a reducing agent.
![]()
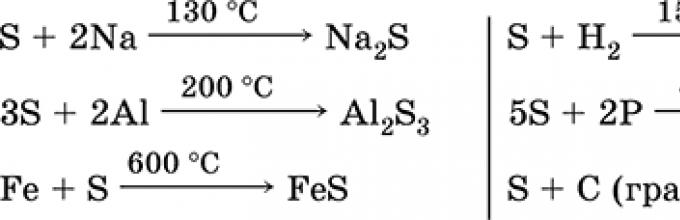
Metals (+):
2Na + S = Na 2 S,
2Al + 3S Al 2 S 3,
Non-metals (+/–)*:
2P + 3S P 2 S 3 ,
S + Cl 2 = SCl 2,
S + 3F 2 = SF 6,
S + N 2 reaction does not occur.
H 2 O (–). sulfur is not wetted by water.
Basic oxides (–).
Acidic oxides (–).
Bases (+/–):
S + Cu(OH) 2 reaction does not occur.
Acids (not oxidizing agents) (–).
Oxidizing acids (+):
S + 2H 2 SO 4 (conc.) = 3SO 2 + 2H 2 O,
S + 2HNO 3 (diluted) = H 2 SO 4 + 2NO,
S + 6HNO 3 (conc.) = H 2 SO 4 + 6NO 2 + 2H 2 O.
In nature, sulfur occurs both in the native state and in the form of compounds, the most important of which are pyrite, also known as iron or sulfur pyrite (FeS 2), zinc blende (ZnS), lead luster (PbS ), gypsum (CaSO 4 2H 2 O), Glauber's salt (Na 2 SO 4 10H 2 O), bitter salt (MgSO 4 7H 2 O). In addition, sulfur is part of coal, oil, as well as various living organisms (as part of amino acids). In the human body, sulfur is concentrated in the hair.
In laboratory conditions, sulfur can be obtained using redox reactions (ORR), for example:
H 2 SO 3 + 2H 2 S = 3S + 3H 2 O,
2H 2 S + O 2 2S + 2H 2 O.
IMPORTANT SULPHUR COMPOUNDS
Hydrogen sulfide (H 2 S) is a colorless gas with a suffocating, unpleasant odor of rotten eggs, poisonous (combines with hemoglobin in the blood, forming iron sulfide). Heavier than air, slightly soluble in water (2.5 volumes of hydrogen sulfide in 1 volume of water). The bonds in the molecule are polar covalent, sp 3-hybridization, the molecule has an angular structure:
Chemically, hydrogen sulfide is quite active. It is thermally unstable; burns easily in an oxygen atmosphere or in air; easily oxidized by halogens, sulfur dioxide or iron(III) chloride; when heated, it interacts with some metals and their oxides, forming sulfides:
![]()
2H 2 S + O 2 2S + 2H 2 O,
2H 2 S + 3O 2 2SO 2 + 2H 2 O,
H 2 S + Br 2 = 2HBr + S,
2H 2 S + SO 2 3S + 2H 2 O,
2FeCl 3 + H 2 S = 2FeCl 2 + S + 2HCl,
H 2 S + Zn ZnS + H 2 ,
H 2 S + CaO CaS + H 2 O.
In laboratory conditions, hydrogen sulfide is obtained by treating iron or zinc sulfides with strong mineral acids or by irreversible hydrolysis of aluminum sulfide:
ZnS + 2HCl = ZnCl 2 + H 2 S,
Al 2 SO 3 + 6HOH 2Al(OH) 3 + 3H 2 S.
Hydrogen sulfide solution in water – hydrogen sulfide water, or hydrosulfide acid . A weak electrolyte, practically does not dissociate in the second stage. How a dibasic acid forms two types of salts −:
sulfides and hydrosulfides
for example, Na 2 S – sodium sulfide, NaHS – sodium hydrosulfide.
Hydrogen sulfide acid exhibits all the general properties of acids. In addition, hydrogen sulfide, hydrosulfide acid and its salts exhibit strong reducing ability.
For example:

H 2 S + Zn = ZnS + H 2, H 2 S + CuO = CuS + H 2 O,
Qualitative reaction to sulfide ion
is interaction with soluble lead salts; In this case, a black precipitate of lead sulfide precipitates:
Pb 2+ + S 2– -> PbS, Pb(NO 3) 2 + Na 2 S = PbS + 2NaNO 3. Sulfur(IV) oxide SO 2 – sp sulfur dioxide, sulfur dioxide - a colorless gas with a pungent odor, poisonous. Acidic oxide. The bonds in the molecule are polar covalent, 2 -hybridization.
Heavier than air, highly soluble in water (in one volume of water - up to 80 volumes of SO 2), forms when dissolved
In terms of acid-base properties, sulfur dioxide exhibits the properties of a typical acid oxide; sulfurous acid also exhibits all the typical properties of acids:
SO 2 + CaO CaSO 3,

H 2 SO 3 + Zn = ZnSO 3 + H 2,

H 2 SO 3 + CaO = CaSO 3 + H 2 O.
In terms of redox properties, sulfur dioxide, sulfurous acid and sulfites can exhibit redox duality (with a predominance of reducing properties). With stronger reducing agents, sulfur(IV) compounds behave as oxidizing agents:
![]()
With stronger oxidizing agents they exhibit reducing properties:
![]()
IN industry sulfur dioxide is obtained:
When burning sulfur:
Roasting of pyrite and other sulfides:
4FeS 2 + 11O 2 2Fe 2 O 3 + 8SO 2,
2ZnS + 3O 2 2ZnO + 2SO 2 .
TO laboratory methods receipts include:
The effect of strong acids on sulfites:
Na 2 SO 3 + 2HCl = 2NaCl + SO 2 + H 2 O;
Interaction of concentrated sulfuric acid with heavy metals:
Cu + 2H 2 SO 4 (conc.) = CuSO 4 + SO 2 + 2H 2 O.
Qualitative reactions to sulfite ion– discoloration of “iodine water” or the action of strong mineral acids:
Na 2 SO 3 + I 2 + 2NaOH = 2NaI + Na 2 SO 4 + H 2 O,
Ca 2 SO 3 + 2HCl = CaCl 2 + H 2 O + SO 2.
Sulfur(VI) oxide SO 3 – sulfur trioxide, or sulfuric anhydride , is a colorless liquid, which at temperatures below 17 ° C turns into a white crystalline mass. Poisonous. Exists in the form of polymers (monomer molecules exist only in the gas phase), the bonds in the molecule are polar covalent, sp 2 -hybridization. Hygroscopic, thermally unstable. Reacts with water with a strong exo-effect. Reacts with anhydrous sulfuric acid to form oleum
![]()
. Formed by the oxidation of sulfur dioxide:,
SO 3 + H 2 O = H 2 SO 4 + SO 3 + H 2 O = H 2 SO 4 + Q
n SO3.:
In acid-base properties it is typical
acid oxide

SO 3 + H 2 O = H 2 SO 4,
![]()
IN SO 3 + CaO = CaSO 4, In terms of redox properties, it acts as a strong oxidizing agent, usually being reduced to SO 2 or sulfites:
pure form has no practical significance; it is an intermediate product in the production of sulfuric acid. Sulfuric acid And – heavy oily liquid without color and odor. Highly soluble in water (with great exo-effect)., which exhibit all the general properties of salts.
Sulfates of active metals are thermally stable, and sulfates of other metals decompose even with slight heating:
Na 2 SO 4 does not decompose,
ZnSO 4 ZnO + SO 3,
4FeSO 4 2Fe 2 O 3 + 4SO 2 + O 2,
Ag 2 SO 4 2Ag + SO 2 + O 2,
HgSO 4 Hg + SO 2 + O 2.
A solution with a mass fraction of sulfuric acid below 70% is usually considered dilute; above 70% – concentrated; a solution of SO 3 in anhydrous sulfuric acid is called oleum (the concentration of sulfur trioxide in oleum can reach 65%). Diluted
sulfuric acid exhibits all the properties characteristic of strong acids:
H 2 SO 4 2H + + SO 4 2– ,
H 2 SO 4 + Zn = ZnSO 4 + H 2,
H 2 SO 4 (diluted) + Cu reaction does not occur,

H 2 SO 4 + CaO = CaSO 4 + H 2 O,
CaCO 3 + H 2 SO 4 = CaSO 4 + H 2 O + CO 2. Concentrated
sulfuric acid is a strong oxidizing agent, especially when heated.

It oxidizes many metals, non-metals, and some organic substances. Iron, gold and platinum group metals do not oxidize under the influence of concentrated sulfuric acid (however, iron dissolves well when heated in moderately concentrated sulfuric acid with a mass fraction of 70%). When concentrated sulfuric acid reacts with other metals, sulfates and sulfuric acid reduction products are formed.
2H 2 SO 4 (conc.) + Cu = CuSO 4 + SO 2 + 2H 2 O,
5H 2 SO 4 (conc.) + 8Na = 4Na 2 SO 4 + H 2 S + 4H 2 O,
H 2 SO 4 (conc.) passivates Fe, Al.
When interacting with non-metals, concentrated sulfuric acid is reduced to SO 2:
5H 2 SO 4 (conc.) + 2P = 2H 3 PO 4 + 5SO 2 + 2H 2 O, 2H 2 SO 4 (conc.) + C = 2H 2 O + CO 2 + 2SO 2. Contact method of receipt
sulfuric acid
consists of three stages:
1) pyrite firing:
![]()
4FeS 2 + 11O 2 2Fe 2 O 3 + 8SO 2 ;
. Formed by the oxidation of sulfur dioxide:,
SO 3 + H 2 O = H 2 SO 4 + 2) oxidation of SO 2 to SO 3 in the presence of a catalyst – vanadium oxide: SO 3 + H 2 O = H 2 SO 4 + Q
3) dissolving SO 3 in sulfuric acid to obtain oleum: SO 3 + H 2 SO 4 (conc.) = H 2 SO 4
Qualitative reaction to sulfate ion
– interaction with the barium cation, resulting in the precipitation of a white precipitate, BaSO 4 .
Ba 2+ + SO 4 2– -> BaSO 4,
1. BaCl 2 + Na 2 SO 4 = BaSO 4 + 2NaCl.
Test on the topic “Sulfur and its compounds”
Sulfur and oxygen are:
a) good conductors of electricity;
b) belong to the subgroup of chalcogens;
2. c) highly soluble in water;
d) have allotropic modifications.
As a result of the reaction of sulfuric acid with copper, you can get:
3. a) hydrogen; b) sulfur;
c) sulfur dioxide; d) hydrogen sulfide.
b) strong oxidizing agent;
c) typical reducing agent;
d) one of the allotropes of sulfur.
4. The mass fraction (in %) of oxygen in sulfuric anhydride is equal to:
a) 50; b) 60; c) 40; d) 94.
5. Sulfur(IV) oxide is an anhydride:
a) sulfuric acid;
b) sulfurous acid;
c) hydrogen sulfide acid;
d) thiosulfuric acid.
6. By what percentage will the mass of potassium hydrosulfite decrease after calcination?
c) potassium hydrosulfite is thermally stable;
7. You can shift the equilibrium towards the direct reaction of oxidation of sulfur dioxide into sulfuric anhydride:
a) using a catalyst;
b) increasing pressure;
c) reducing pressure;
d) reducing the concentration of sulfur oxide (VI).
8. When preparing a solution of sulfuric acid, you must:
a) pour acid into water;
b) pour water into the acid;
c) the order of infusion does not matter;
d) sulfuric acid does not dissolve in water.
9. What mass (in g) of sodium sulfate decahydrate must be added to 100 ml of 8% sodium sulfate solution (density 1.07 g/ml) to double the mass fraction of salt in the solution?
a) 100; b) 1.07; c) 30.5; d) 22.4.
10. To determine the sulfite ion in qualitative analysis, you can use:
a) lead cations;
b) “iodine water”;
c) solution of potassium permanganate;
d) strong mineral acids.
Key to the test
| b, d | V | a, c | b | b | G | b, d | A | V | b, d |
Tasks and exercises on sulfur and its compounds
Chain of transformation
1. Sulfur -> iron(II) sulfide -> hydrogen sulfide -> sulfur dioxide -> sulfur trioxide > sulfuric acid > sulfur(IV) oxide.
3. Sulfuric acid -> sulfur dioxide -> sulfur -> sulfur dioxide -> sulfur trioxide -> sulfuric acid.
4. Sulfur dioxide -> sodium sulfite -> sodium hydrosulfite -> sodium sulfite -> sodium sulfate.
5. Pyrite -> sulfur dioxide -> sulfuric anhydride -> sulfuric acid -> sulfur oxide (IV) -> potassium sulfite -> sulfurous anhydride.
6. Pyrite > sulfur dioxide -> sodium sulfite -> sodium sulfate -> barium sulfate -> barium sulfide.
7. Sodium sulfide -> A -> B -> C -> D -> barium sulfate (all substances contain sulfur; the first, second and fourth reactions are ORR).
| Level A |
1. 6.5 liters of hydrogen sulfide were passed through a solution containing 5 g of sodium hydroxide.
Determine the composition of the resulting solution. Answer.
2. 7 g NaHS, 5.61 g H2S.
Determine the composition of the resulting solution. What mass of Glauber's salt must be added to 100 ml of 8% sodium sulfate solution (the density of the solution is 1.07 g/ml) to double the mass fraction of the substance in the solution?
3. 30.5 g Na 2 SO 4 10H 2 O.
Determine the composition of the resulting solution. To 40 g of a 12% sulfuric acid solution, 4 g of sulfuric anhydride was added. Calculate the mass fraction of the substance in the resulting solution.
4. A mixture of iron(II) sulfide and pyrite, weighing 20.8 g, was subjected to prolonged firing, resulting in the formation of 6.72 liters of gaseous product (o.s.).
Determine the composition of the resulting solution. Determine the mass of the solid residue formed during firing.
5. 16 g Fe 2 O 3.
Determine the composition of the resulting solution. There is a mixture of copper, carbon and iron(III) oxide with a molar ratio of components of 4:2:1 (in the order listed). What volume of 96% sulfuric acid (density 1.84 g/ml) is needed to completely dissolve 2.2 g of such a mixture when heated?
6. 4.16 ml of H 2 SO 4 solution.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. Answer
| . 7.47 g mixture of chromium sulfates (3.92 g) and sodium (3.55 g). |
Level B
1. (problems on oleum)
What mass of sulfur trioxide must be dissolved in 100 g of 91% sulfuric acid solution to obtain 30% oleum?
Solution
According to the problem: m
According to the problem:(H 2 SO 4) = 100 0.91 = 91 g,
(H 2 O) = 100 0.09 = 9 g,
(H 2 O) = 9/18 = 0.5 mol. According to the problem: Part of added SO3 (
1) will react with H 2 O:
H 2 O + SO 3 = H 2 SO 4.
According to the reaction equation:
According to the problem:(SO 3) = (H 2 O) = 0.5 mol.
1 (SO 3) = 0.5 80 = 40 g. According to the problem: Second part SO 3 (

According to the problem: 2) will be used to create a concentration of oleum. Let us express the mass fraction of oleum:
2 (SO 3) = 60 g.
According to the problem: Total mass of sulfur trioxide: According to the problem:(SO 3) = According to the problem: 1 (SO 3) +
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. 2 (SO 3) = 40 + 60 = 100 g.
2. . 100 g SO 3.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. What mass of pyrite must be taken to obtain such an amount of sulfur(VI) oxide that, dissolving it in 54.95 ml of a 91% sulfuric acid solution (density equal to 1.82 g/cm 3), obtain 12.5% oleum? The yield of sulfuric anhydride is considered to be 75%.
3. . 60 g FeS 2.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. To neutralize 34.5 g of oleum, 74.5 ml of a 40% solution of potassium hydroxide (density 1.41 g/ml) is consumed. How many moles of sulfuric anhydride are there per 1 mole of sulfuric acid in this oleum?
4. . 0.5 mol SO3.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. By adding sulfur(VI) oxide to 300 g of 82% sulfuric acid solution, oleum with a mass fraction of sulfur trioxide of 10% is obtained. Find the mass of sulfuric anhydride used.
5. . 300 g SO 3.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. By adding 400 g of sulfur trioxide to 720 g of an aqueous solution of sulfuric acid, oleum with a mass fraction of 7.14% was obtained. Find the mass fraction of sulfuric acid in the original solution.
6. Find the mass of a 64% sulfuric acid solution if adding 100 g of sulfur trioxide to this solution produces oleum containing 20% sulfur trioxide.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction.. 44.4 g of H 2 SO 4 solution.
7. What masses of sulfur trioxide and 91% sulfuric acid solution must be mixed to obtain 1 kg of 20% oleum?
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction.. 428.6 g SO 3 and 571.4 g H 2 SO 4 solution.
8. To 400 g of oleum containing 20% sulfur trioxide, 100 g of a 91% sulfuric acid solution was added.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. Find the mass fraction of sulfuric acid in the resulting solution.
9. . 92% H 2 SO 4 in oleum.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. Find the mass fraction of sulfuric acid in the solution obtained by mixing 200 g of 20% oleum and 200 g of 10% sulfuric acid solution.
10. . 57.25% H2SO4.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. What mass of 50% sulfuric acid solution must be added to 400 g of 10% oleum to obtain an 80% sulfuric acid solution?
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction.. 296.67 g of 50% H 2 SO 4 solution.
. 114.83 g oleum.
1. QUALITATIVE TASKS
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. Colorless gas A with a strong characteristic odor is oxidized by oxygen in the presence of a catalyst into compound B, which is a volatile liquid. Substance B, combining with quicklime, forms salt C. Identify the substances, write the reaction equations.
2. . Substances: A – SO 2, B – SO 3, C – CaSO 4.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. When a solution of salt A is heated, precipitate B is formed. The same precipitate is formed when an alkali acts on a solution of salt A. When an acid acts on salt A, gas C is released, which discolors the solution of potassium permanganate. Identify substances, write reaction equations.
3. . Substances: A – Ca(HSO 3) 2, B – CaSO 3, C – SO 2.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. When gas A is oxidized with concentrated sulfuric acid, a simple substance B, a complex substance C and water are formed. Solutions of substances A and C react with each other to form a precipitate of substance B. Identify the substances, write the reaction equations.
4. . Substances: A – H 2 S, B – S, C – SO 2.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. In the reaction of combining two oxides A and B, liquid at ordinary temperatures, substance C is formed, a concentrated solution of which chars sucrose. Identify substances, write reaction equations.
5. . Substances: A – SO 3, B – H 2 O, C – H 2 SO 4.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction. At your disposal are iron(II) sulfide, aluminum sulfide and aqueous solutions of barium hydroxide and hydrogen chloride. Obtain seven different salts from these substances (without using ORR).
6. When concentrated sulfuric acid acts on bromides, sulfur dioxide is released, and on iodides, hydrogen sulfide is released. Write the reaction equations. Explain the difference in the nature of the products in these cases.
To oxidize 3.12 g of alkali metal hydrosulfite, it was necessary to add 50 ml of a solution in which the molar concentrations of sodium dichromate and sulfuric acid are 0.2 mol/l and 0.5 mol/l, respectively. Determine the composition and mass of the residue that will be obtained when the solution is evaporated after the reaction.. Reaction equations:
2H 2 SO 4 (conc.) + 2NaBr = SO 2 + Br 2 + Na 2 SO 4 + 2H 2 O,
5H 2 SO 4 (conc.) + 8NaI = H 2 S + 4I 2 + 4Na 2 SO 4 + 4H 2 O.
1 See: Lidin R.A."Handbook of general and inorganic chemistry". M.: Education, 1997.
* The +/– sign means that this reaction does not occur with all reagents or under specific conditions.
To be continued