In order to complete and detailed accounting of medicines and medical products in medical organizations of the state healthcare system of the Ulyanovsk region at all stages of their use by medical organizations, I order:
1. Approve the regulations for mandatory measures for recording medicines and medical products in medical organizations (Appendix).
2. Chief physicians of medical organizations of the state healthcare system subordinate to the Ministry of Health of the Ulyanovsk Region:
2.1. Organize work on personalized accounting of medicines and medical products in accordance with the Regulations.
2.2. Conduct a final inventory of medicines and medical products for the institution as of 01/01/2013.
2.3. Develop and approve a procedure for personalized accounting of medicines and medical products, taking into account the specifics of a medical organization.
2.4. Ensure compliance with the confidentiality and security of personal data when processing it in accordance with the legislation of the Russian Federation.
3. Establish that this order comes into force from the date of its signing.
4. I reserve control over the execution of this order.
AND ABOUT. Minister of Health Yu.M. Egorushin
Currently, personalized accounting of medicines makes it possible to increase the safety of pharmacotherapy and optimize the labor costs of medical personnel.
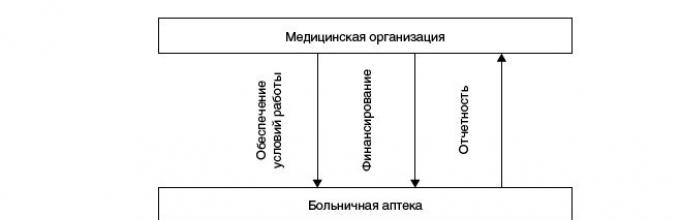
How to build a system in a health care facility pharmacy? The peculiarity of this system is that medications are dispensed personally to each inpatient directly from the hospital pharmacy, bypassing the “storage rooms” of senior nurses of the departments, along a short chain “pharmacy - nursing station”.
Advantages of a personalized medicine accounting system
- exemption of senior nurses from the obligation to receive, store and dispense medications. Concentration of the entire stock of medicines in the pharmacy of the healthcare facility;
- transfer of medical prescriptions from hospital departments directly to the hospital pharmacy through the medical organization’s automated control system;
- pharmacy specialists complete a daily set of medications for each patient and deliver them to the departments;
- personalized accounting of the dispensing of medications during the process of placing an order in a pharmacy;
- production of infusion mixtures in an aseptic box in a pharmacy.
But the main advantages of the unit dose system are:
- savings of up to 30% of a medical organization's allocations for medicines by concentrating the stock of drugs in the pharmacy of a healthcare facility. Interestingly, these American data are fully consistent with the ICH data obtained as a result of the implementation of a system of personalized intrahospital distribution of drugs;
- the possibility of additional pharmaceutical control of drug prescriptions by health care facility doctors and their timely adjustment;
- increasing the safety of pharmacotherapy as a result of placing responsibility for the quality of drugs received by patients on pharmacy staff;
- release of nursing staff to perform their direct functions.
How to implement a personalized drug accounting system
Scheme of personalized intrahospital distribution of drugs
Carrying out this work requires certain technical equipment for hospital pharmacies, the acquisition of which is currently within the capabilities of large hospitals, in the context of modernization.
Firstly, the hospital pharmacy must have modern computer equipment and software built into the automated control system of the healthcare facility and allowing online receipt of medical prescriptions, generation of invoice requirements for medications, keeping records of drugs dispensed, transfer of accounting and reporting documents to the accounting department of the healthcare facility, and also provide the pharmacy with the necessary professional information.
Secondly, medications must be dispensed from the pharmacy in a specific package. This could be a plastic jar containing the daily dose of the drug, for example 2 tablets of diclofenac for 2 doses per day. A large variety of equipment for packaging individual doses of drugs is produced in the United States. There may be packaging in blisters, contour cells, etc. The name of the drug, warning labels and other necessary information are applied to the packaging of drugs using self-adhesive tape. In the case of dispensing single doses in health care facilities, it is advisable to purchase medicines angro (total weight), for example, in a package of 1000 tablets, etc., which creates additional savings for health care institutions.
Thirdly, health care facilities must be equipped with means of delivering individual sets of medications to nursing stations in departments. In large healthcare facilities in the United States, medicines are delivered to departments by pneumatic mail or in gurneys with boxes according to the number of beds in the department. Gurneys are usually used as exchange ones, i.e. in one gurney, drugs are delivered to the department for the current day and left directly in it for a day, and the gurney vacated from the previous day is returned to the pharmacy.
Fourthly, if you transfer the production of infusion mixtures from treatment rooms to a pharmacy, it is necessary to purchase a unit with a laminar flow of sterile air for introducing medications into vials or bags with sterile solutions of solvents or diluents (isotonic solution of sodium chloride, glucose, etc.) under aseptic conditions. Preparing infusion mixtures in a pharmacy instead of treatment rooms improves the quality of the prepared mixtures, eliminates errors made by nursing staff in departments, and ensures strict adherence to the rules for the manufacture and storage of infusion mixtures.
The costs of equipping large healthcare facilities with the necessary equipment are recouped by saving costs on medicines, the costs of which amount to tens of millions of rubles monthly.
Problems of implementing personalized accounting in health care facilities pharmacy
The problem that arises when introducing personalized intrahospital distribution of medicines is, on the one hand, a lack of understanding of the benefits obtained by the management of health care facilities, and on the other hand, the desperate resistance of senior nurses, and sometimes heads of departments, who are often deprived of very significant supplies of medicines in the departments .
Describing the difficulties of implementing the system in question, American experts note that to achieve success, the will of the leader is necessary first of all. Thus, the initiator of the introduction of a system of personalized intrahospital distribution of medicines in 1991 in the ICHB and three other health care facilities was the chairman of the health committee of the administration of the Irkutsk region. The described system operates in these medical organizations to the present day.
And finally, an extremely difficult problem is the selection and training of specialists for the pharmacy of medical institutions, ready to work in new conditions, who understand the role of the pharmacy in organizing rational and safe pharmacotherapy in hospitals.
DEPARTMENT OF HEALTH PROTECTION OF THE POPULATION OF THE KEMEROVSK REGION
ORDER
ON THE IMPLEMENTATION OF PERSONALIZED ACCOUNTING OF MEDICINES IN TREATMENT AND PREVENTIONAL INSTITUTIONS OF THE REGION"
Based on the order of the Ministry of Health and Social Development of the Russian Federation No. 110 dated February 12, 2007 “On the procedure for prescribing and prescribing medicines, medical products (prescriptions and specialized medical nutrition products,” I order:
1. Approve the Regulations on the department of centralized personalized accounting of medicines of a medical institution (hereinafter referred to as the CPMC) (Appendix 1).
2. Approve the application form for medical prescriptions (Appendix 2*).
________________
* Appendix 2 is not provided. - Database manufacturer's note.
3. To the heads of territorial health authorities, chief doctors of central city and district hospitals, state medical and preventive institutions, to ensure timely and high-quality implementation of medical prescriptions, strengthen control over the accounting of medicines and medical products:
3.1. within the period before November 1, 2010, create a central medical treatment center for drugs in subordinate treatment and preventive institutions;
3.2. organize the work of the Central Control Center of Medicines in accordance with the approved Regulations;
3.3. ensure the maintenance of records and reporting, timeliness and completeness of the submission of information to monitor the activities of the Central Administration of Medicines;
3.4. by October 1, 2010, provide step-by-step plans for the implementation of personalized accounting of medicines in medical institutions to the chief regional specialist T.V. Druzhinina by e-mail: [email protected].
4. Director of KOMIAC L.E. Isakova by 10/15/2010, ensure the development of software and monitoring of the activities of the central control center with the provision of information to the department on a monthly basis no later than the 5th day of the month following the reporting one.
5. Responsibility for the execution of the order should be assigned to the first deputy head of the department O.V. Seledtsova, acting. Deputy Head of the Department V.N. Chegodaev.
6. I reserve control over the execution of this order.
Head of department
V.K.TSOY
Appendix 1. REGULATIONS ON THE DEPARTMENT OF CENTRALIZED PERSONALIZED DRUG CONSUMPTION ACCOUNTING
Annex 1
to the department order
public health protection
Kemerovo region
dated September 22, 2010 N 1088
1. General Provisions
1.1. This Regulation defines the main tasks, functions, rights and responsibilities of the department of centralized personalized accounting of drug consumption (CPMC).
1.2. OTsPU LS in its activities is guided by orders of the Ministry of Health and Social Development of the Russian Federation, other legal and methodological documents.
1.3. OTsPU LS is a structural unit of a medical institution, created and liquidated by order of the chief physician.
1.4. Management of the work of the department is entrusted to the head of the department, to whose position a person with a higher medical or pharmaceutical education is appointed by order of the chief physician.
1.5. The staffing level and structure of the department are approved by the chief physician within the existing staffing table, based on the characteristics of the department’s work and the need to ensure work around the clock.
1.6. OTsPU LS carries out its activities in cooperation with the pharmacy of the medical institution and all structural divisions around the clock.
2. Main tasks of the department
The main tasks of the OCPU are:
Optimization of drug provision for patients in healthcare facilities through personalized distribution of drugs;
Organization of centralized administration of antibiotics by injection;
Improving the accounting of medicines and medical products;
Ensuring reporting and monitoring the implementation of assigned tasks, the department for centralized personalized accounting of drug consumption performs the following functions:
Carries out requests for medical appointments from ward and guard nurses of the departments.
Receives drugs at the pharmacy upon request - an invoice drawn up on the basis of prescription sheets.
Carries out the layout of ampoule and tablet medications (in accordance with the entries in the prescription sheets).
Delivers ampoules of drugs for intravenous infusions, laid out personally for patients, to the treatment nurse.
Delivers tablet medications to each patient in the ward in accordance with the doctor’s prescription and indicating the rules of administration.
Performs intramuscular injections in the wards, according to the notes on the doctor's prescription sheets.
Informs medical units about the availability of medications.
Complies with the sanitary-epidemiological regime and pharmaceutical procedures in accordance with established rules.
Keeps records and prepares reports on drug consumption in the prescribed manner.
To perform the assigned functions, the OCPU nurse is obliged and has the right:
In a timely manner, in accordance with established rules, perform intramuscular injections to patients in hospital departments.
Timely and efficiently, in accordance with established requirements, arrange medications in departments according to assignment sheets and distribute ampoule medications for intravenous infusions to procedural nurses.
Distribute tablet forms directly to patients in the wards in a timely manner, in accordance with the rules for taking medications.
Check expiration dates of medicines.
Follow the rules for storing medicines in accordance with established requirements.
Follow the technological rules for intramuscular injections.
Complete current documentation in accordance with established requirements.
Submit daily requests to the head of the Medical Center for Medicines for replenishment of supplies.
Comply with sanitary requirements in the workplace.
Participate in the inventory of inventory items in accordance with the instructions of the head of the department.
Systematically improve your qualifications and study specialized literature.
Strictly comply with internal labor regulations, fire safety and labor protection rules.
In all difficult cases, receive advice from the head of the department related to the distribution of medications.
Take advantage of regulated breaks to maintain performance throughout the entire work shift.
Make suggestions to improve the work of the department.
Participate in events held at the hospital.
To perform the functions assigned to it, the department of centralized personalized accounting of drugs is given the right to:
Request and promptly receive from departments sheets of medical prescriptions and lists of discharged patients.
Monitor the correct completion of applications for the availability of the drug dose, frequency of administration, and indicative course.
Inform senior nurses of departments about mistakes made by ward nurses when filling out applications from the Central Medical Center for Medicines.
To perform functions and exercise rights, the OCPU interacts with:
With the hospital pharmacy on issues of providing the department with medications and consumables.
With medical departments regarding the receipt of applications for medical prescriptions.
With the central accounting department on the submission of accounting and reporting documentation.
With the economic department on issues of supplying office supplies, as well as on issues of carrying out repairs in the premises assigned to the Central Control Center of Medicines.
Department employees bear individual responsibility:
For the correct placement of intramuscular injections, placement of medications and timely delivery of tablet forms to patients.
For the sanitary condition of your workplace.
For the safety of inventory items.
For compliance with internal labor regulations, fire safety and labor protection.
Algorithm for the action of a nurse at the Central Clinical Hospital when distributing medicines to departments
I. Preparation for distribution
1. Before starting work in the drug distribution room, the workplace is wet cleaned in accordance with current standards.
2. Before starting the layout, perform hygienic treatment of the hand in a hygienic manner.
3. Put on special clothes located in the layout room.
4. Place the necessary equipment for work on the layout table: clip bucks (packaging bags), wax paper, a container for tablet forms, scissors, as well as a pen and a marker.
5. Place packages of medications on the desktop according to pharmacological groups or alphabetically.
II. Distribution
The layout is carried out according to the algorithm:
1. Check that the applications are filled out correctly, that they contain the dose of the drug, the frequency of its use, the course of treatment and the signature of the ward nurse.
2. Distribution of ampoule dosage forms should be carried out in packaging bags (clipbucks) indicating the department, full name. patient, ward N.
3. Distribution of tablet dosage forms should be carried out in containers for tablet forms, indicating the department, full name. patient, room number, time of admission (morning, lunch, evening, night).
4. Place the distributed medications in special trays for each compartment.
Attention:
When distributing, pay attention to the expiration dates of medications;
When working, follow safety rules.
Upon completion of distribution, clean the workplace.
1. Arrange the remaining drugs in storage cabinets according to pharmacological groups.
2. Remove packaging material.
3. Carry out a wet cleaning of the desktop.
Note:
Prohibited:
1. The presence of unauthorized persons in the room for drug distribution.
2. Use cell phones.
3. Eating, smoking and having extraneous conversations.
4. Store personal belongings in a specially designated place.
The ward nurse works with appointment sheets only in the document processing room<**>.
________________
<**>The regulations are subject to modification in each health care facility depending on local conditions. For the effectiveness of personalized accounting of drug consumption, it is necessary to consider the centralized distribution of all forms of medicines, medical products, and specialized medical nutrition products.
The Regulations should include the procedure for maintaining records and reporting, a mechanism for determining the need, generating an application, informing doctors about the availability and receipt of medicines, and the procedure for issuing requirements.
This order does not apply to emergency departments, intensive care units, and maternity wards.
The administration of narcotic, psychotropic, potent and toxic substances is carried out in accordance with previously established procedures.
Personalized accounting of medicines and medical devices: approach to implementation Sergey Dmitrievich Gusev, head of the information department of the Federal Center for Cardiovascular Surgery (Krasnoyarsk) Grounds for maintaining personalized accounting of drugs and medical devices “Procedure for maintaining personalized accounting in the field of compulsory health insurance” . Order of the Ministry of Health and Social Development of the Russian Federation dated January 25, 2011 No. 29n. 4. Personalized recording of information about medical care provided to insured persons includes the collection, processing, transfer and storage of the following information: ... 10) medical services provided to the insured person and medications used; ... “On approval of the general principles of construction and operation information systems and the procedure for information interaction in the field of compulsory health insurance.” Order of the Federal Compulsory Medical Insurance Fund dated 04/07/11 No. 79 Table 10 List of information on medical care provided to insured persons in the field of compulsory health insurance...20. Used medications... Grounds for maintaining personalized records of medicines and medical supplies “Rules for the provision of paid medical services by medical organizations.” Decree of the Government of the Russian Federation of October 4, 2012 No. 1006 29. The Contractor provides the consumer (legal representative of the consumer) at his request and in a form accessible to him information: ... about the medicines and medical devices used in the provision of paid medical services, including about their expiration dates (warranty periods), indications (contraindications) for use. Goals of personalized accounting of drugs and medical devices 1. Determination of actual costs for each case of medical care - the main element of managing the tariff policy of a medical organization and the industry as a whole 2. Analysis of the use (frequency of use) of drugs and medical devices - a mechanism for monitoring the implementation of medical care standards 3. Planning procurement of drugs and medical devices in case of changes in the structure of treated patients 4. Obtaining basic information for analyzing the causes of side effects (lack of effect), adverse reactions when using drugs (including blood products) and medical drugs 5. Formation of an invoice ) for payment of medical care in the provision of paid medical services 6. Basis for conducting pharmacoeconomic research Personalized accounting of medicines and medical devices - problems “at the start”: The name of the product when posted, as a rule, does not correspond to its “consumer” name Papaverine g/x , injection solution 2% - 2ml, ampoules No. 10 Papaverine hydrochloride, d/in solution. , 2%, amp., 2 ml, No. 10 Armavir biological factory Sol.Papaverini hydrochloridi 2% - 2.0 IV cannula with injection. port Vasofix Certo 20G 33 mm, Germany IV catheter (with port) Vasofix Safety, 20G, 33 mm, clip, Malaysia Venous catheter, 33 mm The product characteristics of drugs and MI do not describe their “consumer” and “accounting” properties the code (if available) does not contain a description of the characteristics of the drug and MI. Only donor blood and its components can be reliably identified by the barcode. Nomenclator of drugs - structure Name according to the receipt document ATC - anatomical group ATC - therapeutic group ATC - pharmacological group INN Types of accounting (PKU, accounting, sum, serial, etc.) Release form 1 Release form 2 Release form 3 Amount of active substance, DDD, etc. Amount of active substance, DDD, etc. Amount of active substance, DDD, etc. Units of measurement and conversion factors Units of measurement and conversion factors Units of measurement and conversion factors Characteristics of the series Characteristics of the series Characteristics of the series Identification number (barcode) Identification number (barcode) Identification number (barcode) Nomenclator of medicinal products - structure ATC classification group Trade name on Russian Name in Latin INN Dosage form Release form Series Expiration date Minimum unit dispensed from the pharmacy Amount of active substance Unit of measurement of active substance Established daily dose (DDD) Belonging to vital and essential drugs Accounting group Accounting type (by series, total accounting) Belonging to PKU Units of accounting (measurement) in a pharmacy, in a department, in the accounting department Conversion factors (consumer packaging → secondary packaging) Belonging to indirect accounting (“daily consumption”) The nomenclator allows you to describe drugs in detail for the purposes of their further use, accounting and analytical reporting. Nomenclator of medicinal products - structure ATC classification group Fields of the GRLS reference book Trade name in Russian 1. Unique identifier (UI) 2. Registration authorization number Name in Latin 3. Registration date INN 4. Expiration date of the registration Dosage form of the certificate Release form 5. Date of cancellation of the registration certificate Series 6. Legal entity in whose name the registration certificate was issued Expiration date Minimum unit of release 7. Country of location of the legal entity in whose name the registration certificate was issued from the pharmacy 8. Trade name of the medicinal product Amount of active substance 9. International generic or chemical Unit of measurement of the active name of the substance 10. Forms of release Established daily dose (DDD) 11. Information about the stages of production 12 . Barcodes of consumer packaging Belonging to vital and essential drugs 13. Regulatory documentation Accounting group 14. Pharmacotherapeutic group Type of accounting (by series, total accounting) Belonging to PKU Accounting units (measurement) in the pharmacy, in the department, in the accounting department Conversion factors ( consumer packaging → secondary packaging) Belonging to indirect accounting (“day consumption”) The nomenclator allows you to describe drugs in detail for the purposes of their further use, accounting and analytical reporting. Medicine nomenclator in the 1C: Hospital Pharmacy system Classifiers of medical devices “On approval of the nomenclature classification of medical devices.” Order of the Ministry of Health of the Russian Federation dated June 6, 2012 No. 4n GOST ISO 15225-2011 “Nomenclature. Nomenclature of data on medical devices for information exchange.” Entered into force on January 1, 2013. Directories of the NSI registry of the Ministry of Health of the Russian Federation Classifier of medical devices (medical products and medical equipment) (Date of last change - 09.11.2011) Classifier of areas of medical application of medical products (Date of last change - 10.11.2011) Classification of medical products depending on the potential risk of use (Date of last change – 11/10/2011) List of medical equipment products in accordance with the application form for the purchase of medical equipment (Date of last change – 12/09/2013) Nomenclator of medical devices - structure Name according to the receipt document Accounting group Product group 1 Product group 2 Product subgroup 1 Product subgroup 2 Unified name 1 Unified name 2 Trade name + Country + Manufacturer + General technical. characteristics Trade name + Country + Manufacturer + General technical. characteristics Manufacturer's catalog number + Size + Technical characteristics Manufacturer's catalog number + Size + Technical characteristics Series, expiration date Series, expiration date Identification number (barcode) Identification number (barcode) GOST ISO 15225-2011 “Nomenclature. Nomenclature of data on medical devices for information exchange.” Effective from January 1, 2013. Nomenclator of medical products - structure Accounting group Product group 1 Product group 2 Product subgroup 1 Product subgroup 2 Product categories Unified (according to GOST ISO 15225-2011) - name 1 widely used Trade name + medical definitions Country + Manufacturer + products covering General technical. characteristics of various products that have a common area Manufacturer’s catalog number + Size + application Technical characteristics (for example, “medical equipment”, “Consumables”, Series, expiration date, etc.). Identification number (bar code) Unified name 2 Trade name + Country + Manufacturer + General technical. characteristics Manufacturer's catalog number + Size + Technical characteristics Series, expiration date Identification number (bar code) GOST ISO 15225-2011 “Nomenclature. Nomenclature of data on medical devices for information exchange.” Entered into force on January 1, 2013. Nomenclator of medical devices - structure Accounting group Product group (according to GOST ISO 15225-2011) - products that have the same or similar scope of application (for example, “Heart valve prostheses”, “ Vascular prostheses”, “Stents”, “Suture material”) Product group 1 Product group 2 Product subgroup 1 Product subgroup 2 Unified name 1 Unified name 2 Trade name + Country + Manufacturer + General technical. characteristics Trade name + Country + Manufacturer + General technical. characteristics Manufacturer's catalog number + Size + Technical characteristics Manufacturer's catalog number + Size + Technical characteristics Series, expiration date Series, expiration date Identification number (barcode) Identification number (barcode) GOST ISO 15225-2011 “Nomenclature. Nomenclature of data on medical devices for information exchange.” Entered into force on January 1, 2013. Nomenclator of medical products - structure Accounting group Product group 1 Product group 2 Subgroup of goods 1 Subgroup of goods 2 Unified Unified name 2 Subgroups formed by name 1 based on the distinctive characteristics of the groups Trade name + Country + Manufacturer + goods, definitely General technical. characteristics that determine their use. Catalog number Various subgroups of the manufacturer + Size + of the same group not Technical characteristics can be Series, expiration date interchangeable. (e.g. “Sterile” – Identification number “Non-sterile”). (barcode) Trade name + Country + Manufacturer + General technical. characteristics Manufacturer's catalog number + Size + Technical characteristics Series, expiration date Identification number (bar code) GOST ISO 15225-2011 “Nomenclature. Nomenclature of data on medical devices for information exchange.” Effective from January 1, 2013. Nomenclator of medical devices - structure Accounting group Product group 1 Product group 2 Product subgroup 1 Product subgroup 2 Unified name 1 Unified name 2 Trade name + Country + Manufacturer + General technical. characteristics Trade name + Country + Manufacturer + General technical. characteristics Unified name of the product without indicating the trade name Manufacturer's catalog number + Size + brand and/or product Technical characteristics of the mark and/or manufacturer. (for example: “Venous cannula”, Series, expiration date “Bone wax”) Manufacturer catalog number + Size + Technical characteristics Identification number (barcode) Identification number (barcode) Series, expiration date GOST ISO 15225-2011 "Nomenclature. Nomenclature of data on medical devices for information exchange.” Entered into force on January 1, 2013. Nomenclator of medical devices - structure Product type (according to GOST ISO 15225-2011) - individual medical products for individual use, indicating the brand (trademark, manufacturer). Accounting group Product group 1 Product group 2 Subgroup of goods 1 Subgroup of goods 2 Unified name 1 Unified name 2 Trade name + Country + Manufacturer + General technical. characteristics Trade name + Country + Manufacturer + General technical. characteristics Manufacturer's catalog number + Size + Technical characteristics Manufacturer's catalog number + Size + Technical characteristics Series, expiration date Series, expiration date Identification number (barcode) Identification number (barcode) GOST ISO 15225-2011 “Nomenclature. Nomenclature of data on medical devices for information exchange.” Entered into force on January 1, 2013. Nomenclator of medical devices - structure Product model, Accounting group containing the name (brand name, Product group 1 Product group 2 product catalog number, size, etc.), Product subgroup 1 Product subgroup 2 used by the manufacturer for product identification. Unified Unified name 2 Filled in if the name 1 medical product Trade name + Trade name + has a model range Country + Manufacturer + Country + Manufacturer + General technical. characteristics General technical characteristics Manufacturer's catalog number + Size + Technical characteristics Manufacturer's catalog number + Size + Technical characteristics Series, expiration date Series, expiration date Identification number 1 (bar code) Identification number 2 (bar code) GOST ISO 15225-2011 “Nomenclature” . Nomenclature of data on medical devices for information exchange.” Entered into force on January 1, 2013. Nomenclator of medical devices - example of a description of a medical device Cardiac biological valve. Carpentier Edwards Perimount aort., size 23, USA Consumable material Prosthetic heart valve Suture material Aortic Mitral Valve aortic biological Valve aortic mechanical Carpentier -Edwards PERIMOUNT, USA Medtronic 3f Enable QH12345, 23 mm QH12346, 25 mm Series R-123, 12/31/2014 Series R-124, 12/31/2015 2000123456789 2000123456790 GOST ISO 15225-2011 “Nomenclature. Nomenclature of data on medical devices for information exchange.” Entered into force on January 1, 2013. Identification of medicinal products name of the active substance series, expiration date Method Conditions for the use of the dispenser in pharmacies Storage conditions name of the drug dosage company logo mark of originality companymanufacturer country (city) manufacturer dosage form Registration certificate number quantity of the drug in the package barcode MR 64-03-004-2004 Methodological recommendations “Graphic design of medicines. General requirements". Approved by the order of the Department of Industrial and Innovation Policy in the Medical and Biotechnological Industry dated April 27, 2004 No. 15/11-9. Identification of medicinal products GTIN (Global Trade Item Number) Code of the National Organization - a member of EAN International Registration number of the enterprise Product code within the enterprise Control number “The enterprise chooses the product code independently, highlighting the classification characteristics of the product at its discretion...” OST GISLS N 91500.05.0002 -2001 “State information standard for medicinal products. Basic provisions". Approved by order of the Ministry of Health of the Russian Federation on March 26, 2001 No. 88 Identification of medicinal products ≠ “The enterprise chooses the product code independently, highlighting the classification characteristics of the product at its discretion...” OST GISLS N 91500. 05.0002-2001 “State information standard for medicinal products. Basic provisions". Approved by order of the Ministry of Health of the Russian Federation on March 26, 2001 No. 88 Identification of medicinal products "UNISKAN / GS1 RUS" (http://www.gs1ru.org) is a voluntary non-profit non-governmental organization operating on the territory of the Russian Federation. Consists of member enterprises using a unified standard for product numbering and bar coding. The standard is created and supported by the largest planetary non-profit organization GS1, which is in charge of standardizing the accounting and bar coding of logistics units. Identification of GRLS drugs: the same bar code, but different dosages in Russia today does not allow. effectively identify a medicinal product at the product type level. “Introduction in Russia of Data Matrix codes as a method of unique identification of medicinal products” “World experience in creating systems for automatic identification and traceability of medicinal products” Kirill Maksimovich Starostin, business development manager of NP “Lingvapharm” International conference “Modernization of information processes in healthcare (including the sphere of circulation of medicines and medical devices)” December 11-12, 2013, Moscow Barcode labeling of medical devices can be done in various ways. Labeling using a barcode for domestic medical products is not provided: “ Unified sanitary-epidemiological and hygienic requirements for goods subject to sanitary-epidemiological supervision (control)” (Approved by the Decision of the Customs Union Commission of May 28, 2010 No. 299). Chapter II. Section 18. Part 5 “Requirements for consumer labeling of medical devices and medical equipment and user information.” “...labeling and packaging are the exclusive competence and responsibility of the product manufacturer.” Letter of the Department of State Control of Medicines, Medical Products and Medical Equipment of the Ministry of Health of the Russian Federation dated February 24, 2004 No. 293-22/34 Identification of medical devices Labeling in the HIBCC standard (Health Industry Business Communications Council - Coordination Council of Medical Device Manufacturers) Primary stroke code check number identifier of the packaging level (0 – primary, etc.) product number according to the manufacturer's catalog manufacturer code (code of the enterprise that generated the label) Universal product number Secondary barcode check number check number of the primary barcode series number expiration date quantity identifier of quantity/expiration date/lot or series number The health industry supplier labeling standard: for patient safety and unique device identification (HIBC / SLS / UDI) ANSI/HIBC 2. 4 – 2013 http://www.hibcc.org/publication/view/supplier-labeling-standard/ Identification of medical devices GS1 QR Code GS1 DataMatrix Labeling in the GS1 standard. Elements of the code: Indicator: The leftmost digit is indicates to the GS1 DataBar that the code is a GS1 Global Item Identifier (GTIN). The second digit is the packaging level identifier (0 – primary, etc.) Manufacturer code: Globally unique number assigned to the company by the GS1 association. GS1 128 Product reference number: part of the GTIN intended to identify product products from the manufacturer's catalogue. Control number Identification of medical devices by GTIN for personalized accounting tasks does not give anything... Identification of donor blood and its components 7241212123456020 Control number Unique number of a given unit of product within a given donation Unique donation number The last two digits of the year in which the donation was carried out Code of the organization that carried out donation Code of an object of republican subordination Sign used to comply with the requirements of GOST 30743 National standard of the Russian Federation GOST R 52938-2008 “Donor blood and its components. Containers with preserved blood or its components. Marking". Date of introduction - 03/01/2009 Identification of donor blood and its components Identification of drugs and medical devices Problem solution: own identifier (barcode) associated with a detailed description of the drug or medical device, focused on the tasks of the medical organization Saving drowning people is the work of the drowning people themselves! The number of registered and labeled medicines and medical devices in the Federal State Budgetary Institution "Federal Center for Agricultural Medicine" for 2011 - 2013 1 2 3 1 - the number of secondary packages of medicines; 2 – number of MI units; 3 – number of packages X 100 Price of one label – 3.3 kopecks. Personalized accounting of medicines and medical products. Pharmacy. Incorporation of medicines and medical products into IS "1C: Pharmacy of a medical institution" Registration of incoming goods in strict accordance with the delivery note Correlating the registered goods with the corresponding position of the nomenclator Printing a label with a bar code and sticking it on the secondary packaging of medicines and medical devices Automatic export of data on registered goods Medicines and medical supplies in IS "1C: Accounting of a budgetary institution" Work with departmental applications generated in IS "1C" Transfer of medicines and medical supplies from the pharmacy warehouse to the department's warehouse according to applications with automatic export of data to MIS Personalized accounting of medicines and medical supplies. Department. Excluded (not taken into account) positions Groups of accounted drugs and medical supplies A. Medicines and medical supplies of “direct” accounting, when in the provision of medical care each unit of consumed drugs and (or) medical supplies used during planned and emergency drug therapy is taken into account; Donor blood and its components, preparations from donor blood, blood replacement solutions; Implantable devices (stents, prostheses, pacemakers, etc.); Introducers, catheters, single-use guides; Expensive medical devices used in transfusion and infusion therapy (oxygenators, single-use devices and devices, including for connecting polymer lines) B. “Indirectly” taken into account drugs and medical devices Syringes and blood transfusion systems; Solutions Suture material Disinfectants Sterile and non-sterile disposable medical products (wipes, gloves, gowns, sheets, diapers, etc.) Accounting for consumed medical devices and blood products during the operation “direct recording” of medical devices in the operating room is carried out “on-line” Accounting for consumable drugs and medical supplies in the department Medicines and medical supplies received by the head nurse of the department are transferred to the nursing stations Accounting for consumable drugs and medical supplies in the department Accounting for consumable drugs and medical supplies in the department For each item at any time, balances can be monitored Accounting for consumable drugs and medical supplies in the department Accounting for consumed drugs and medical supplies in the department The “Daily Consumption” mode is focused on “indirectly” taken into account drugs and medical products Accounting for donor blood and its components For each case of blood transfusion, a protocol is filled out Accounting for donor blood and its components Detailed information is accumulated for each blood component Accounting for donor blood and its components components Detailed information is accumulated for each hemocomponent. Results of personalized accounting of drugs and medical devices: Journal of registration of transfusion of transfusion media; automatic generation of all necessary accounting and reporting forms according to Order of the Ministry of Health of the USSR dated April 10, 1980 No. 1030 Results of personalized accounting of drugs and medical devices: Card of clinical and economic accounting contains a list of all medicinal products and medical devices spent on the treatment of a patient Results of personalized accounting of medicinal products and medical devices: The write-off control card contains a list of all medicinal products and medical devices written off for patients in a given period of time Results of personalized accounting of medicinal products and medical devices: The expiration date control card contains a list of all medicinal products and medical devices with given minimum shelf life for the current date Results of personalized accounting of drugs and medical devices: Information support for a clinical pharmacologist Possibility of conducting pharmacoeconomic analysis based on both purchased and consumed drugs Conclusion Personalized accounting of drugs and medical devices is an important element of accounting and planning expenses of medical organizations and the industry as a whole for the provision of medical care. It is necessary to expand the State Register of Medical Devices and Organizations engaged in the production and manufacture of medical devices of Roszdravnadzor in terms of increasing the characteristics of medical devices. For personalized accounting of medicines and medical devices, it is necessary to have their detailed description. A unified federal information resource is needed that allows you to obtain information about medicines and medical devices by their unique identifier ( barcode) or On consumer (secondary) packaging of medicines and medical devices there must be a barcode with more information (PDF 417, Data Matrix) To solve the problem of personalized accounting at the industry level, the presence of a unique identifier of medicines and medical devices is a necessary condition Thank you! Your questions? Sergey Dmitrievich Gusev, head of the information department of the Federal State Budgetary Institution "Federal Center for Cardiovascular Surgery" (Krasnoyarsk) [email protected]